Recent studies in our laboratory and others point to an emerging role for neuromuscular control of bone homeostasis. Indeed, given the abundant innervation of bone, even modest interruptions in neuromuscular signaling pathways have potential to greatly influence skeletal homeostasis. In this context, neuropeptides including calcitonin-gene related peptide, Substance P, and Neuropeptide Y, Y1, and Y2, as well as neurotransmitters such as norepinephrine, dopamine and serotonin have all been implicated in the regulation of bone remodeling. Therefore, our group is focused on elucidating the neuromuscular signaling pathways and tissue interactions that control trabecular bone remodeling and homeostasis.
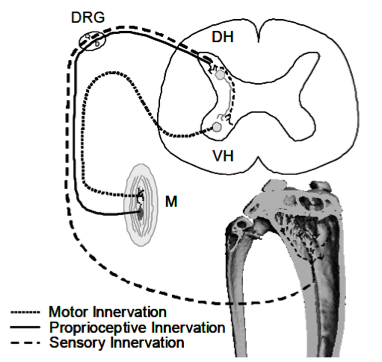 Simplified schematic illustrating sensory and motor innervation of appendicular muscle and bone. Muscles (M) receive motor neuron input via the ventral horn (VH), but muscle proprioception and bone sensory neurons transmit signals via the dorsal horn (DH). The anatomical juxtaposition of these systems at the DH and dorsal root ganglia (DRG) allows for crosstalk with potential to alter bone homeostasis within the metaphyseal trabecular compartment.
Simplified schematic illustrating sensory and motor innervation of appendicular muscle and bone. Muscles (M) receive motor neuron input via the ventral horn (VH), but muscle proprioception and bone sensory neurons transmit signals via the dorsal horn (DH). The anatomical juxtaposition of these systems at the DH and dorsal root ganglia (DRG) allows for crosstalk with potential to alter bone homeostasis within the metaphyseal trabecular compartment.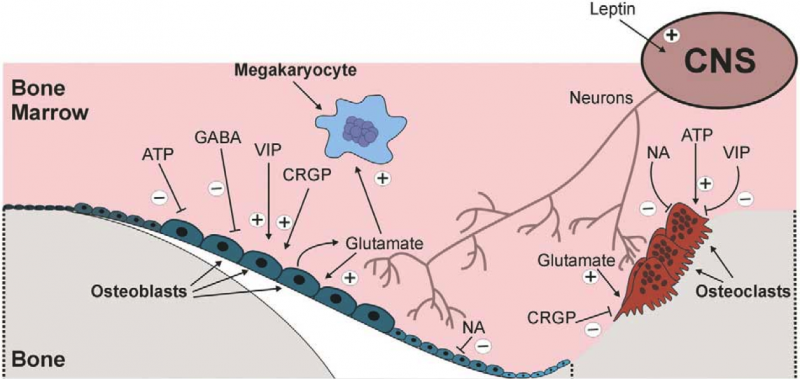 Neuroskeletal Signaling Pathways
Neuroskeletal Signaling PathwaysSome of our current projects in this area include...
Contralateral Bone Loss Following Ipsilateral Nerve Injury: Evidence for a Neuronal Skeletome
Peripheral nerve injury (PNI) is a frequent complication of orthopaedic trauma that can lead to muscle atrophy, sensory impairment and chronic pain. Bone loss has also been associated with nerve damage and has been attributed to reductions in mechanical loading due to paralysis, immobilization, and/or inactivity, but in the absence of muscle atrophy or motor damage the mechanism of bone loss is unclear. In this context, previous studies in our group have demonstrated that PNI leads to significant bone loss without concomitant muscle atrophy. Given these observations and our data suggesting a role for sensory nerves in bone remodeling, we hypothesized that sensory nerves may play a role in bone loss following PNI. To explore this hypothesis, we assessed bone loss in the proximal tibia following PNI in TRPV1 KO mice, which have altered pain responses due to sensory nerve deficits. Mice also underwent gait analysis to quantify peak ground reaction forces (GRF) as a control for possible effects of PNI on altered weight bearing. Consistent with our previous studies, PNI resulted in significant bone loss of the ipsilateral tibia in WT mice within 4 weeks. TRPV1 KO mice also demonstrated an equivalent decrease in tibial metaphyseal bone. Surprisingly, WT mice also experienced a -47.0% decrease in BV/TV of the proximal tibia of the contralateral limb, while contralateral bone loss in TRPV1 KO mice was attenuated by 30.5% (Fig. 1). Further, there were no bone changes in the ipsilateral forelimb, demonstrating the absence of a systemic effect due to PNI. Finally, peak GRF following PNI were no different, providing evidence that weight-bearing was not altered by nerve injury. Taken together, these results indicate that ipsilateral bone loss following PNI is a consequence of altered neuronal signaling and is unrelated to changes in mechanical loading. However, given that ipsilateral bone loss in WT and TRPV1 KO mice following PNI was equivalent, the TRPV1 receptor does not appear to play a significant role in the ipsilateral osteopenia. However, the magnitude of contralateral bone loss in this model is a novel and unexpected observation and, in the absence of changes in weight bearing, raises the provocative question of how the left tibia so closely mirrored bone loss in the right tibia. In this context, the attenuation of contralateral bone loss in TRPV1 KO mice would be consistent with a role for sensory nerve pathways in bilateral “cross-talk” between ipsilateral and contralateral tibia. Furthermore, the findings in our model display surprising similarity to reports of contralateral osteopenia in patients with chronic regional pain syndrome (CRPS). Given the recent findings that demonstrate a direct role of sensory nerves in skeletal homeostasis, we speculate that sensory innervation of bone maps skeletal morphology in much the same way that sensory nerves map the dermatome. Given this linkage, we speculate that nerve injury induced alterations to a neuronal “Skeletome” at one level has potential to alter bone homeostasis at distant sites.
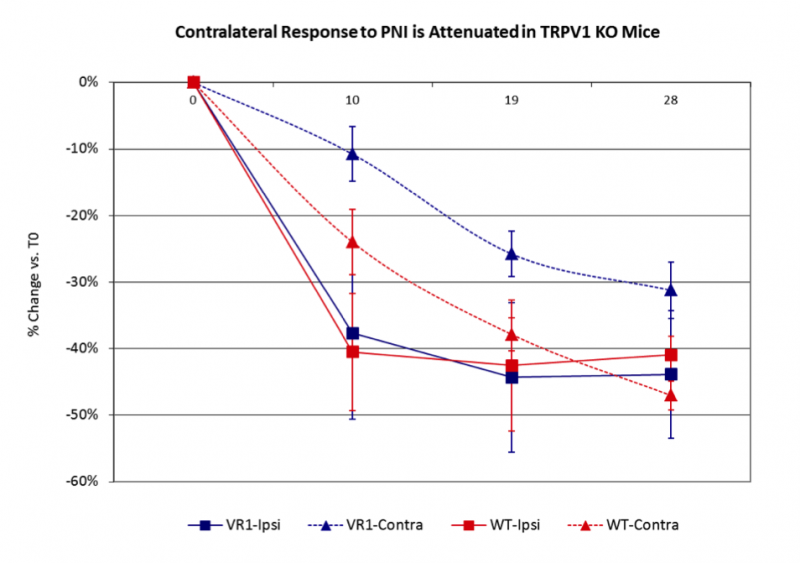 Figure 1. PNI in WT and TRPV1 KO mie led to significant loss of cancellous bone in both the ipsi and contralateral proximal tibiae, suggesting homeostatic linkage between the two sites. Suprisingly, contralateral bone loss was partly attenuated in TRPV1 KO mice, suggesting that sensory innervation may play a role in the bilateral cross talk. Finally, there were no bone changes in the ipsilateral humerus (data not shown), providing evidence that PNI did not trigger systemic bone loss.
Figure 1. PNI in WT and TRPV1 KO mie led to significant loss of cancellous bone in both the ipsi and contralateral proximal tibiae, suggesting homeostatic linkage between the two sites. Suprisingly, contralateral bone loss was partly attenuated in TRPV1 KO mice, suggesting that sensory innervation may play a role in the bilateral cross talk. Finally, there were no bone changes in the ipsilateral humerus (data not shown), providing evidence that PNI did not trigger systemic bone loss.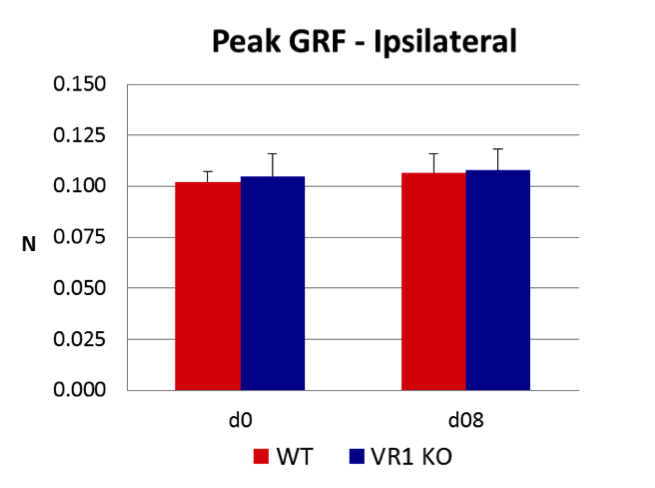 Figure 2. There were no changes in peak GRF between WT and TRPV1 KO mice after PNI, providing evidence that weight bearing was not altered by nerve injury.
Figure 2. There were no changes in peak GRF between WT and TRPV1 KO mice after PNI, providing evidence that weight bearing was not altered by nerve injury.Trabecular Bone Homeostasis is Modulated by Neuromuscular Proprioception.
The rate and magnitude of trabecular bone loss in the tibia following transient calf paralysis with botulinum toxin A (BTxA) exceeds that induced by hindlimb suspension but is comparable to sciatic neurectomy (SCX) or spinal cord injury (SCI). As BTxA induced calf paralysis induces only a mild gait dysfunction compared, we reasoned that reduction of gait induced bone deformations arising secondary to motor impairment were not the sole driver of acute bone loss. Because BTxA also impairs sensory signaling arising from the paralyzed limb, we speculated that BTxA-induced sensory disruption was causally linked to acute bone resorption following transient muscle paralysis. To address this question we used high-resolution in vivo microCT to assess bone morphology of the proximal tibia metaphysis in two genetic models of disrupted sensory signaling; the vanilloid (capsaicin) receptor knockout mouse (VR1), and the radiation induced Sprawling mouse (Swl +/-). VR1 mice have impaired nociception (with normal proprioception and motor function), while Swl +/- mice display a hindlimb proprioceptive neuropathy (with normal nociception and motor function). Wild type VR1 and SWL mice demonstrated significant loss of BV/TV at d 5 and d 12 following calf paralysis, with VR1 KO mice demonstrating the same bone loss as controls. In contrast, Swl +/- mice demonstrated no bone loss at d 5 and significantly attenuated bone loss at d 12. These initial findings implicate neuromuscular proprioception as a novel pathway that directly modulates trabecular bone homeostasis.
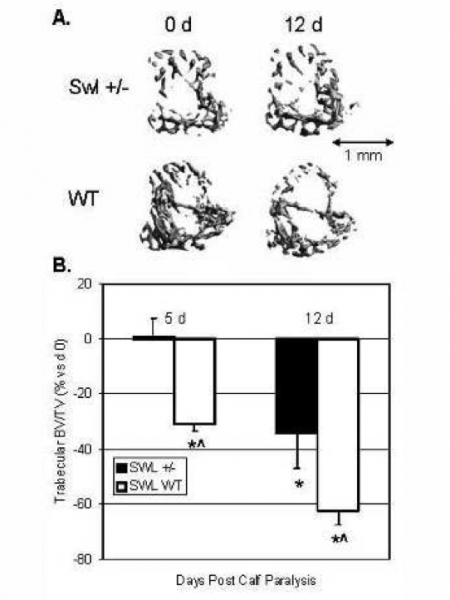
Representative 3-D microCT images of trabecular bone within the proximal tibia metaphysis of representative Swl +/- and WT littermate mice at 5 and 12 d post calf paralyssis (vs d 0; B). In direct support of our general hypothesis, Swl +/- demonstrate a low trabecular BV/.TV phenotype and a delayed and reduced loss of trabecular bone following transient muscle paralysis (*, p<0.05 vs d 0, ^, p0.05 vs KO at same time).
