The main focus of our laboratory involves investigation of genes that play important roles in the development and maintenance of the mammalian skeletal system. We are especially interested in how changes in gene expression lead to skeletal disorders or malignancies in mice and human. To achieve our goals of understanding the molecular mechanisms underlying skeletal diseases in human, we use various techniques including molecular biology, cellular biology and mouse genetics.
Research Projects
Regulation of Interphalangeal Joint Development
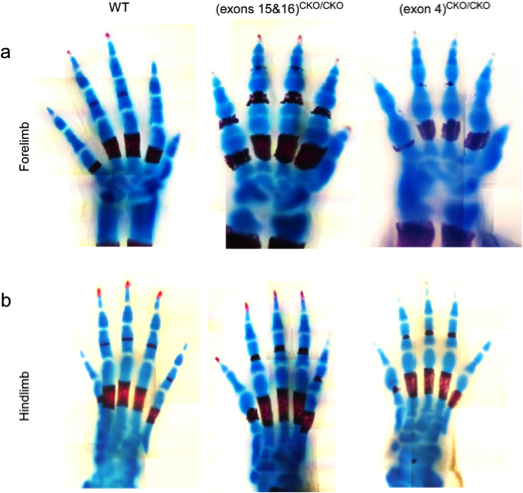 The development of interphalangeal joints consists of three phases: interzone formation, cavitation, and subsequent morphogenesis. We have recently found that ESET is required in order for forming joints to proceed through the step of cavitation. We observed that the timing of ESET expression is critical to joint development using Prx1-cre mice as the deleter strain for mating with the mice containing the floxed ESET allele. Forelimbs of the resultant knockout mice show a lack of cavitated interphalangeal joints, whereas hindlimbs appear to be unaffected (figure). We are continuing to investigate the specific role ESET plays in the development of interphalangeal joints.
The development of interphalangeal joints consists of three phases: interzone formation, cavitation, and subsequent morphogenesis. We have recently found that ESET is required in order for forming joints to proceed through the step of cavitation. We observed that the timing of ESET expression is critical to joint development using Prx1-cre mice as the deleter strain for mating with the mice containing the floxed ESET allele. Forelimbs of the resultant knockout mice show a lack of cavitated interphalangeal joints, whereas hindlimbs appear to be unaffected (figure). We are continuing to investigate the specific role ESET plays in the development of interphalangeal joints.
Epigenetic regulation of articular joints
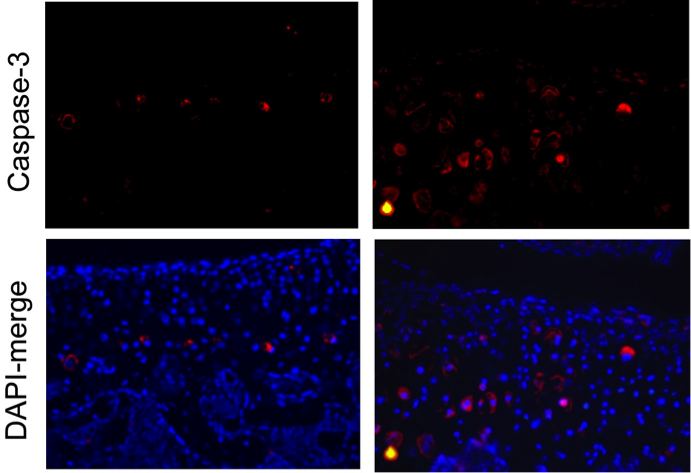
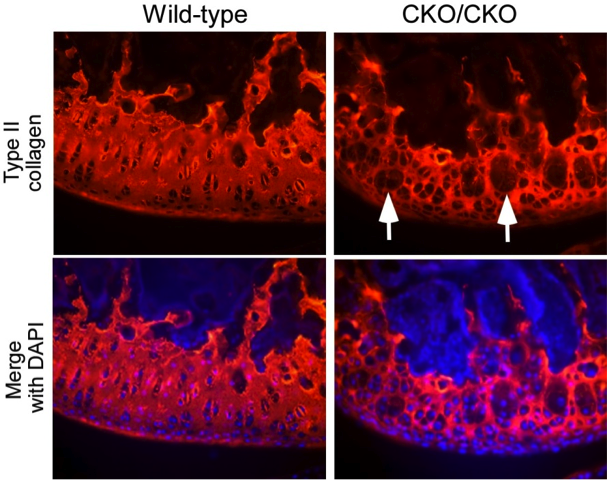 During skeletogenesis and joint formation, chondrocytes invariably follow two separate developmental paths. Growth plate chondrocytes display transient behavior, characterized by rapid proliferation and terminal differentiation, whereas articular chondrocytes are slow in turnover and phenotypically stable. In certain disease states, however, articular chondrocytes fail to maintain their latent phenotype and become mitotically active, ultimately proliferating and expressing proteins such as type-X collagen, alkaline phosphatase and MMP-13 that are indicative of chondrocyte terminal differentiation. This is a process reminiscent to the growth of long bones through endochondral ossification. We recently demonstrated that the protein ESET histone methyltransferase is transiently upregulated in growth plate chondrocytes to function as a ‘gate keeper’ in preventing entry into terminal differentiation, thus ESET knockout results in premature hypertrophy of growth plate chondrocytes and early physeal closure during skeletal development. We have subsequently observed that deletion of the ESET gene is also associated with ectopic hypertrophy of articular chondrocytes, overt signs of chondrogenic terminal differentiation, and early onset of articular cartilage degeneration.
During skeletogenesis and joint formation, chondrocytes invariably follow two separate developmental paths. Growth plate chondrocytes display transient behavior, characterized by rapid proliferation and terminal differentiation, whereas articular chondrocytes are slow in turnover and phenotypically stable. In certain disease states, however, articular chondrocytes fail to maintain their latent phenotype and become mitotically active, ultimately proliferating and expressing proteins such as type-X collagen, alkaline phosphatase and MMP-13 that are indicative of chondrocyte terminal differentiation. This is a process reminiscent to the growth of long bones through endochondral ossification. We recently demonstrated that the protein ESET histone methyltransferase is transiently upregulated in growth plate chondrocytes to function as a ‘gate keeper’ in preventing entry into terminal differentiation, thus ESET knockout results in premature hypertrophy of growth plate chondrocytes and early physeal closure during skeletal development. We have subsequently observed that deletion of the ESET gene is also associated with ectopic hypertrophy of articular chondrocytes, overt signs of chondrogenic terminal differentiation, and early onset of articular cartilage degeneration.
Development of trabecular bones
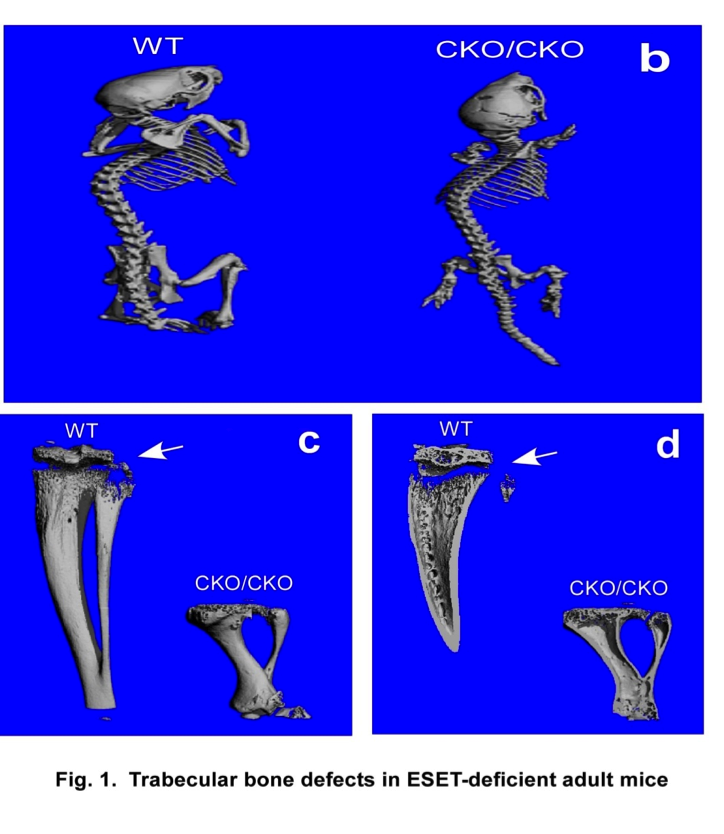 Bone formation takes place through two distinct processes: endochondral ossification involving a cartilage anlagen and intramembranous ossification by which bones form directly from condensations of mesenchymal cells without a cartilage intermediate. Intramembranous ossification is limited to the formation of certain flat bones, such as the pelvis, skull, scapulae, and clavicles, whereas endochondral ossification forms the remainder of the skeleton. Bone formation and remodeling are coupled temporally and spatially, and both are controlled by the interplay of growth signals, mechanical stress, hormones and specific transcription factors. Despite recent advances in our understanding of bone formation, epigenetic regulation of bone development by histone methylation enzymes remains unclear. Phenotypic analysis of mesenchymal specific knockout of the ESET protein demonstrates impaired development of trabecular bones in mice. We are currently studying the cellular and genetic mechanisms behind this abnormality.
Bone formation takes place through two distinct processes: endochondral ossification involving a cartilage anlagen and intramembranous ossification by which bones form directly from condensations of mesenchymal cells without a cartilage intermediate. Intramembranous ossification is limited to the formation of certain flat bones, such as the pelvis, skull, scapulae, and clavicles, whereas endochondral ossification forms the remainder of the skeleton. Bone formation and remodeling are coupled temporally and spatially, and both are controlled by the interplay of growth signals, mechanical stress, hormones and specific transcription factors. Despite recent advances in our understanding of bone formation, epigenetic regulation of bone development by histone methylation enzymes remains unclear. Phenotypic analysis of mesenchymal specific knockout of the ESET protein demonstrates impaired development of trabecular bones in mice. We are currently studying the cellular and genetic mechanisms behind this abnormality.
Osteochondral graft storage media
 Osteochondral allograft transplantation is a common treatment option to address large articular defects, providing functional restoration of the affected joint. Traditionally “fresh” osteochondral allografts were utilized within days of harvest, resulting in good long term clinical outcomes. Recently, due to increased graft demand and concerns regarding disease transmission, increased screening has prolonged the time period from tissue harvest to clinical use. This prolonged storage has led to decreased graft integrity and in vivo performance. In particular, the chondrocyte viability decreases drastically after storage, leading to a diminished population of cells capable of maintaining articular cartilage integrity within the grafted tissues. Our lab is currently studying the optimal storage conditions to preserve osteochondral graft integrity and cell viability.
Osteochondral allograft transplantation is a common treatment option to address large articular defects, providing functional restoration of the affected joint. Traditionally “fresh” osteochondral allografts were utilized within days of harvest, resulting in good long term clinical outcomes. Recently, due to increased graft demand and concerns regarding disease transmission, increased screening has prolonged the time period from tissue harvest to clinical use. This prolonged storage has led to decreased graft integrity and in vivo performance. In particular, the chondrocyte viability decreases drastically after storage, leading to a diminished population of cells capable of maintaining articular cartilage integrity within the grafted tissues. Our lab is currently studying the optimal storage conditions to preserve osteochondral graft integrity and cell viability.
EWS-Fli1 fusion protein and Ewing’s sarcoma
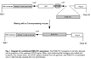 Ewing’s sarcoma (a bone tumor) is characterized by the recurrent t(11;22) chromosomal translocation and the resultant EWS-Fli1 fusion protein. At the present time, it is not clear what cell lineage the tumor arises from. With transgenic mouse lines in which the EWS-Fli1 gene can be selectively expressed in a specific cell lineage or tissue through breeding with various Cre-mouse lines (Fig. 1), we aim to establish a mouse model of Ewing’s sarcoma that can be used as a platform for cutting-edge therapeutic treatments.
Ewing’s sarcoma (a bone tumor) is characterized by the recurrent t(11;22) chromosomal translocation and the resultant EWS-Fli1 fusion protein. At the present time, it is not clear what cell lineage the tumor arises from. With transgenic mouse lines in which the EWS-Fli1 gene can be selectively expressed in a specific cell lineage or tissue through breeding with various Cre-mouse lines (Fig. 1), we aim to establish a mouse model of Ewing’s sarcoma that can be used as a platform for cutting-edge therapeutic treatments.
Chondrogenesis and histone modification enzymes
 During endochondral bone formation, proliferating chondrocytes undergo an orderly transition into hypertrophic chondrocytes for eventual cell death and replacement by osteoblasts. We have found that the ESET histone methyltransferase, originally cloned by our laboratory, is expressed in pre-hypertrophic chondrocytes and is required for proper development of mouse embryos (Fig. 2). ESET regulates chromatin structure and controls gene expression through methylation of histone H3 at lysine 9. We are currently investigating how a lack of ESET protein causes premature hypertrophic differentiation of chondrocytes in mouse embryos and young adults.
During endochondral bone formation, proliferating chondrocytes undergo an orderly transition into hypertrophic chondrocytes for eventual cell death and replacement by osteoblasts. We have found that the ESET histone methyltransferase, originally cloned by our laboratory, is expressed in pre-hypertrophic chondrocytes and is required for proper development of mouse embryos (Fig. 2). ESET regulates chromatin structure and controls gene expression through methylation of histone H3 at lysine 9. We are currently investigating how a lack of ESET protein causes premature hypertrophic differentiation of chondrocytes in mouse embryos and young adults.
