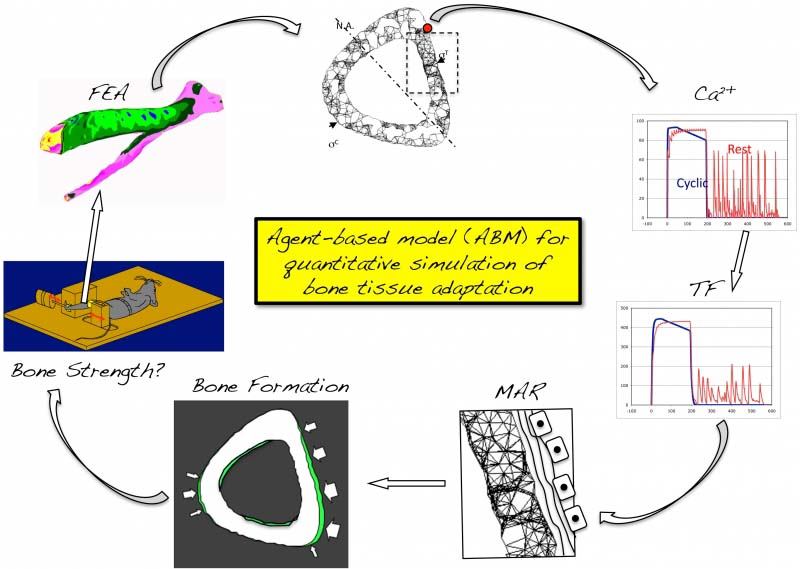
We have developed an agent-based modeling (ABM) framework for the quantitative simulation of bone adaptation. The model explores how real-time second messenger signaling (e.g. Ca2+) and subsequent activation of transcription factors (e.g., NFAT, c-fos, b-Catenin) within the bone cellular network regulates the proliferation and differentiation of precursor cells, and ultimately, bone matrix secretion by differentiated osteoblasts.
Upon calibration, the ABM has proved capable of quantitatively simulating bone formation induced in young and senescent animals by a wide variety of mechanical stimuli. The model has identified deficits in cell signaling that underlie the muted responsiveness of the senescent skeleton and directly enabled the discovery of an intervention (supplementation with low-dose Cyclosporin A) that enabled the senescent skeleton to respond robustly to mechanical stimuli and equivalent to adaptation induced in young adult animals. ABMs have also enabled the optimization of loading protocols whereby the required loading ‘effort’ could be reduced ~100 fold yet enabled in a 2-fold increase in bone adaptation.
In effect, ABMs have enabled novel insights into mechanotransduction in bone and have predicted the successful design of loading protocols and treatments that are counterintuitive, extrapolative and could not have been conceived without the model. Importantly, the modular framework of the ABM permits unprecedented future opportunities to explore bone mechanotransduction across vast temporal and spatial scales, from second messengers to pathway activation and interactions, gene expression, and cell and tissue adaptation, and ultimately, in quantitatively describing bone strength.

