Investigations Into Collagen: The Skeleton’s Scaffold
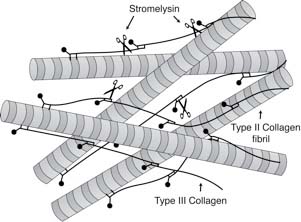 Collagen is a protein polymer that underlies the strength of almost all tissues in our bodies. It forms the essential framework of our bones and cartilage, for example.
Collagen is a protein polymer that underlies the strength of almost all tissues in our bodies. It forms the essential framework of our bones and cartilage, for example.
The Burgess Chair Research Program, led by David R. Eyre, Ph.D., professor of Orthopædic & Sports Medicine and the Ernest M. Burgess Endowed Chair for Orthopædic Investigation, is studying defects in collagen. With this research, he and his colleagues will develop a better understanding of how diseases of bones and cartilage, like osteoporosis and arthritis, develop. This knowledge can then be used in developing treatments to maintain or help repair these tissues.
Genetics and environment
Osteoporosis and bone loss are insidious problems affecting over 45 million adults in the United States today. We know that genetics (heritable factors) play a major role in the development of osteoporosis, but the genetics are complex. Large-scale, worldwide population genetic screens are being used to identify osteoporosis-associated genes.
The latest results, however, are surprising. Only a few genes have emerged, and none are strongly associated with fracture risk. These results raise another possibility that environmental and lifestyle factors known to affect bone quality may influence genetic risk.
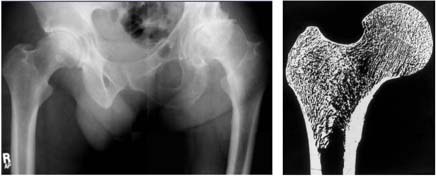
Collagen and brittle bones
We know that the biochemistry of the collagen framework in adult bones varies markedly among individuals, but we don’t know why. Some scientists suspect it is related to environmental factors (diet, vitamin D levels, zinc intake, etc.) that, over a lifetime of bone turnover, may affect the quality of the tissue.
Scientists are investigating this possibility by using molecular methods to understand how altered bone collagen chemistry is linked to brittle bones. Working in collaboration with scientists here and at other universities, we have studied rare forms of a heritable and severe brittle bone disease, osteogenesis imperfecta, which is caused by known defects in collagen formation. In investigating osteogenesis imperfecta, we have discovered clues about processes that may cause bone collagen to deteriorate in normal skeletons. One day, we hope that this research will help us predict which patients are at great risk for developing osteoporosis.
Joint injuries and collagen changes
Osteoarthritis is another disabling condition, one that affects almost 55% of the adult population in the United States. Like osteoporosis, heritability is a major factor in determining who might develop osteoarthritis, but thus far, only genes with weak links to osteoarthritis risk have been found. Again, the genetic risk may be linked to environmental factors.
Reaction to injury may help predict disease. For instance, people have different capacities to repair wear-and-tear joint injuries, and these differences appear to determine who is at greatest risk for potential join failure.
There may be other indicators too. Studies reveal that the earliest stages of osteoarthritis are marked by molecular-level changes in the collagen fabric of join cartilage. In addition, these studies show differences in the underlying biochemistry of join cartilage that resemble bone changes we’ve noted in other studies.
Interested in research
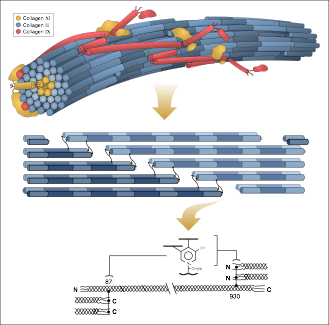 Basic research, research that will help us to better understand the chemistry of collagen, for instance, is critical to finding new treatments for disabling conditions such as osteoporosis and osteoarthritis.
Basic research, research that will help us to better understand the chemistry of collagen, for instance, is critical to finding new treatments for disabling conditions such as osteoporosis and osteoarthritis.
If you are interested in the research done by Dr. Eyre and his colleagues in the Burgess Chair Research Program, please email him at deyre@u.washington.edu. Also review many of the recent publications by Dr. Eyre and his colleagues here.
