The recently published article ESET histone methyltransferase is essential to hypertrophic differentiation of growth plate chondrocytes and formation of epiphyseal plates--written by Orthopaedic faculty members Drs. Liu Yang, PhD, Howard Chansky, MD, Steven Bain, PhD, and Russell Fernandes, PhD--was recognized in Faculty 1000 as being an article of special significance in the field of cartilage biology.
Faculty 1000 is the publisher of four unique services that support and inform the work of life scientists and clinicians. It is well known to provide a layer of expert opinion and guidance on published articles.
A summary of the article provided on Faculty 1000’s website is shown below:
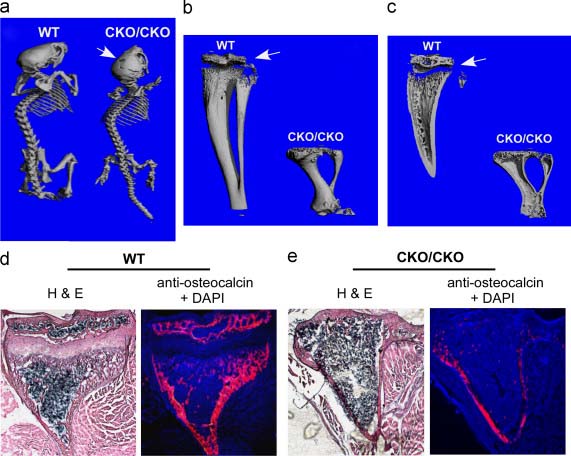
Chondrocyte hypertrophy is a major driver of longitudinal bone growth during development, but hypertrophy of articular chondrocytes contributes to pathogenic changes in osteoarthritis (OA). While numerous transcription factors are known to regulate chondrocyte hypertrophy {1}, the epigenetic mechanisms regulating this process remain relatively unknown. In the present article, Yang and colleagues note transient up-regulation of ESET, an H3K9 histone methyltransferase, in prehypertrophic chondrocytes. To explore the possibility that ESET regulates hypertrophic differentiation, conditional Eset knockout mice {2} were crossed to a Prx1-Cre driver line {3}, to produce offspring in which critical Eset exons 15 and 16 are deleted in developing limb buds. Prx1-ESET-null mice display a variety of skeletal defects at birth, including shortened scapula, humerus, radius, ulna and digits in the forelimbs, with milder effects in the hindlimbs. Interestingly, bones formed through intramembranous ossification, including the skull, were also affected, suggesting that ESET might also function in the osteoblast lineage. Disorganized Prx1-ESET-null growth plates displayed areas of apparent ectopic hypertrophy: cells outside of the hypertrophic zone stained positive for collagen X, which is normally restricted to hypertrophic chondrocytes, and apoptotic cells were more widely distributed across the growth plate. Postnatal bone growth was also affected by loss of ESET, likely due to the absence of epiphyseal plates. Taken together, these findings suggest that ESET normally restricts the temporal and spatial pattern of hypertrophic differentiation in developing growth plates. To address a possible mechanism through which ESET might function, the authors assessed whether ESET functionally interacts with Runx2 and HDAC4, two known regulators of hypertrophy {4}. Tagged ESET and Runx2 proteins formed a complex with HDAC4, and ESET repressed Runx2-mediated activation of the osteocalcin2 promoter in a reporter assay. This effect was reversed upon pharmacological inhibition of histone deacetylases, suggesting that ESET cooperates with HDAC4 to repress Runx2-mediated transcriptional activation. Prx1-ESETnull mice share important phenotypic similarities with mice bearing conditional loss of Ihh in chondrocytes, including ectopic hypertrophy and loss of the epiphyseal plates {5}, suggesting that ESET may regulate Ihh expression in prehypertrophic chondrocytes to spatially mediate hypertrophic differentiation in the growth plate. In line with this hypothesis, the abundance of Ihh-positive cells is reduced in Prx1-ESET-null mice. While these mice have an intriguing phenotype, several issues remain to be elucidated. For example, Prx1-Cre becomes active around E9.5 in the forelimb {3}, far before growth plates are apparent. Thus, ESET-dependent changes in the early limb mesenchyme may contribute to the phenotypes described here, including effects on the osteoblast lineage. Furthermore, both the direct transcriptional targets of ESET and the epigenetic consequences of ESET loss in limb mesenchyme (i.e. changes in H3K9 methylation) remain unknown. Nevertheless, these findings have important implications for understanding how transcriptional regulators, including epigenetic factors, regulate endochondral ossification during embryonic development and postnatal skeletal growth, as well as for understanding the molecular aspects of OA progression, including hypertrophy of articular chondrocytes.
More information regarding the research being done by Dr. Liu Yang can be found at his website.
*image taken from ESET histone methyltransferase is essential to hypertrophic differentiation of growth plate chondrocytes and formation of epiphyseal plates. Yang L, Lawson KA, Teteak CJ, Zou J, Hacquebord J, Patterson D, Ghatan AC, Mei Q, Zielinska-Kwiatkowska A, Bain SD, Fernandes RJ, Chansky HA. Dev Biol. 2013 Aug 1;380(1):99-110. doi: 10.1016/j.ydbio.2013.04.031. Epub 2013 May 4. PMID: 23652029